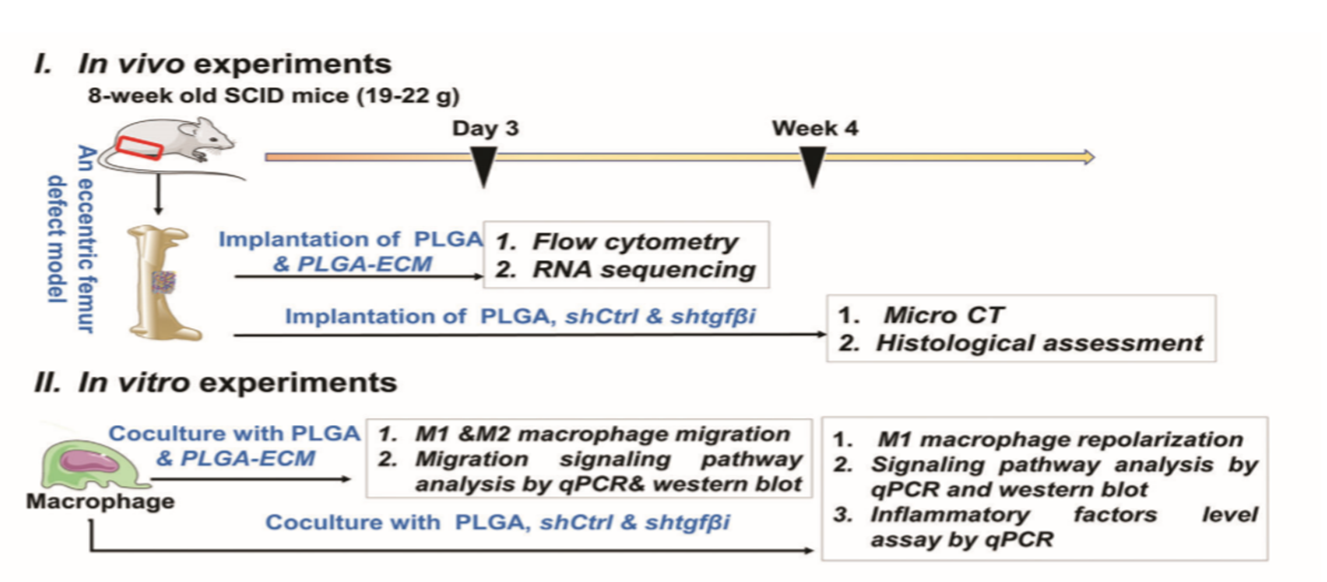
人脐带间充质干细胞(hUCMSC)在指导巨噬细胞行为方面具有关键的免疫调节特性。本文中,首次通过hUCMSC衍生的细胞外基质(PLGA-ECM)修饰的3D打印聚丙交酯-乙交酯(PLGA)支架通过物理脱细胞的简便组织工程技术制备包裹多种可溶性细胞因子的PLGA-ECM支架。在改良的SCID小鼠异位移植模型中,PLGA-ECM不仅可以减轻异物反应,而且可以通过增加M2巨噬细胞的积累来改善骨骼再生。此外,研究表明,PLGA-ECM改善了M2巨噬细胞的积累可能归因于M2巨噬细胞的迁移和复极化M1巨噬细胞对M2表型的表达,其通过TGFβI依赖性方式由涉及整合素𝜷7,整合素α9和整合素𝜷1的多个整合素信号传导途径介导。这项研究提出了一种有效的聚合物骨架表面修饰策略,以通过增加M2巨噬细胞的积累来启动组织再生并抵抗炎症反应。
董世武,教授,博士,第三军医大学,累计参与发表SCI 135篇,主要从事骨生物学与组织工程骨软骨及相关生物材料研究。研究方向为:1)间充质干细胞(MSCs)成骨、软骨分化的分子机制、生物界面修饰对种子细胞的分化机制及其在组织工程骨软骨修复中的应用;2)软骨下骨失衡致创伤性骨关节炎的发病机理研究;3)破骨细胞融合机制及其与骨质疏松症、骨肿瘤转移的相关研究。提出“基质微环境骨重建”概念,并阐明其组织、细胞及分子机制;创立“软骨内成骨基质微环境” 修复方案,并阐释其分子机制;率先将 “破骨”概念引入修复微环境,提出“调控破骨功能,成骨+血管+塑形”的整体修复理念;建立基于基质材料-细胞界面的三维成骨微环境,阐释了其对多类修复细胞行为的影响及调控机制。团队创立了“干细胞富集”等三大关键技术,参与了M-TEB等三项国家标准制定。建立了基质依赖型TEB、软骨内成骨模式TEB体系;参与了种子细胞及基质依赖型TEB标准的制定;为膜诱导技术TEB的临床大量应用提供了理论基础。
Human umbilical cord mesenchymal stem cells (hUCMSCs) have a pivotal immunomodulatory property on directing macrophage behaviors. Herein, for the first time, 3D-printed poly(lactide-co-glycolide) (PLGA) scaffolds modified with hUCMSC-derived extracellular matrix (PLGA-ECM) are prepared by a facile tissue engineering technique with physical decellularization and contain multiple soluble cytokines with a sustainable release profile. The PLGA-ECM not only attenuates the foreign body response, but also improves bone regeneration by increasing the accumulation of M2 macrophages in an improved heterotopic transplantation model of SCID mice. Furthermore, the PLGA-ECM scaffolds with the knockdown of transforming growth factor-𝜷-induced protein (TGF𝜷I/𝜷ig-H3) demonstrate that M2 macrophage accumulation improved by the PLGA-ECM could be attributed to increasing the migration of M2 macrophages and the repolarization of M1 macrophages to M2 phenotype, which are mediated by multiple integrin signaling pathways involving in integrin 𝜷7, integrin 𝜶9, and integrin 𝜷1 in a TGF𝜷I-dependent manner. This study presents an effective surface modification strategy of polymeric scaffolds to initiate tissue regeneration and combat inflammatory response by increasing M2 macrophage accumulation.
Dong Shiwu, MD, Ph.D., Third Military Medical University, has published 135 SCI papers, mainly engaged in bone biology and tissue engineering osteochondral and other related biomaterial research. The research directions are: 1) the molecular mechanism of mesenchymal stem cells (MSCs) osteogenic and cartilage differentiation, the differentiation mechanism of biological interface modification on seed cells and its application in tissue engineering osteochondral repair; 2) subchondral bone imbalance caused Research on the pathogenesis of traumatic osteoarthritis; 3) Research on the mechanism of osteoclast fusion and its correlation with osteoporosis and bone tumor metastasis. Put forward the concept of “matrix microenvironment bone reconstruction” and clarify its tissue, cell and molecular mechanism; create a repair plan for “intracartilage osteogenic matrix microenvironment” and explain its molecular mechanism; take the lead in introducing the concept of “osteogenesis” into the repair microenvironment , Put forward the overall repair concept of “regulating osteoclast function, osteogenesis + blood vessel + shaping”; established a three-dimensional osteogenic microenvironment based on the matrix material-cell interface, and explained its influence on the behavior of multiple types of repair cells and the regulation mechanism. The team created three key technologies such as “stem cell enrichment”, and participated in the formulation of three national standards such as M-TEB. Established the matrix-dependent TEB and intrachondral osteogenesis model TEB system; participated in the formulation of seed cell and matrix-dependent TEB standards; provided a theoretical basis for the large-scale clinical application of membrane induction technology TEB。
DOI:10.1002/adhm.202000353
(周梦瑒)