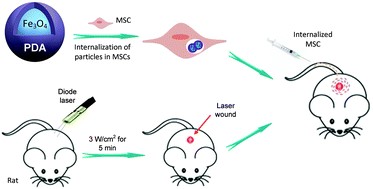
具有许多独特特性(包括生物相容性和超顺磁特性)的磁性纳米粒子(NPs)通常用于医学中。近年来,已对磁性NPs进行了广泛的MSC标记研究。尽管一些研究表明,NPs可以在体外被内化到细胞中,然后使用外部磁场(EMF)引导至靶组织,但没有报道表明在不存在EMF的情况下具有类似的作用。因此,有必要开发具有无毒涂层的NP,以满足体外有效地被MSC吸收的生理化学需求。近年来,一些研究致力于开发用于MSC标记的新型NP涂层,例如聚吡咯,聚苯胺,氨基聚乙烯醇和聚多巴胺(PDA),以改善Fe3O4 NP 的生物相容性。例如,PDA显示出出色的生物相容性和生物降解性,而不会在体内引起长期毒性。因此,Fe3O4@PDA比Fe3O4具有更低的毒性和更好的生物相容性。并且更适合临床治疗。
近日,吉林大学中日联谊医院姜金兰团队成功地合成了Fe 3 O 4 @PDA NP,可以被MSC有效地吸收。发现Fe 3 O 4 @PDA NPs具有高度相容性,且对MSC的毒性极小。植入激光损伤的皮肤后,与单独的MSC治疗相比,Fe 3 O 4 @PDA NPs增强了MSC的募集并改善了抗炎和愈合能力。数据表明,Fe 3 O 4的施用PDA NP标记的MSCs进入损伤部位,可以增强SDF-1 / CXCR4轴,将是治疗烧伤创面的一种新型治疗策略。展示了在临床相关动物模型中注射标记的MSC来治疗皮肤伤口的安全性和可行性。还表明,体内静脉内注射标记的MSC可有效促进伤口修复。
Magnetic nanoparticles (NPs), which possess numerous unique characteristics, including biocompatibility and superparamagnetic properties, are commonly applied in medicine.Magnetic NPs have been studied extensively for MSC labeling in recent years.Although some studies have revealed that NPs can be internalized into cells in vitro and then guided to target tissues using an external magnetic field (EMF),there have been no reports showing a similar effect in the absence of EMFs. Therefore, it is necessary to develop NPs with non-toxic coatings that meet the physiochemical need for efficient cellular uptake by MSCs in vitro. In recent years, several studies have focused on the development of novel NP coatings for MSC labeling, such as polypyrrole, polyaniline, amino-polyvinyl alcohol, and polydopamine (PDA), to improve the biocompatibility of Fe3O4 NPs. For example, PDA shows excellent biocompatibility and biodegradability, without inducing long-term toxicity in vivo. Therefore, Fe3O4@PDA has lower toxicity and better biocompatibility than Fe3O4 and is more suitable for clinical treatment.
Recently, Professor Jiang jinlan from China-Japan Union Hospital of Jilin University successfully synthesized Fe3O4@PDA NPs, which could be efficiently taken up by MSCs. The Fe3O4@PDA NPs were found to be highly compatible with minimal toxicity to the MSCs. After implantation at the laser injured skin, the Fe3O4@PDA NPs enhanced the recruitment of MSCs and improved the anti-inflammatory and healing ability compared with the MSC treatment alone. The data suggest that administration of the Fe3O4@PDA NP-labeled MSCs to the injured site, which can enhance the SDF-1/CXCR4 axis, would be a novel therapeutic strategy for the treatment of burn wounds. It showed the safety and feasibility of injecting the labeled MSCs in a clinically relevant animal model for treating cutaneous wounds. It also showed that in vivo intravenous injection of the labeled MSCs was efficient in promoting wound repair.
DOI: 10.1039/c9bm00242a
吴硕