|
通过对Fe3O4/MC涂层施加磁驱动机械刺激,加强MC3T3-E1细胞的成骨分化
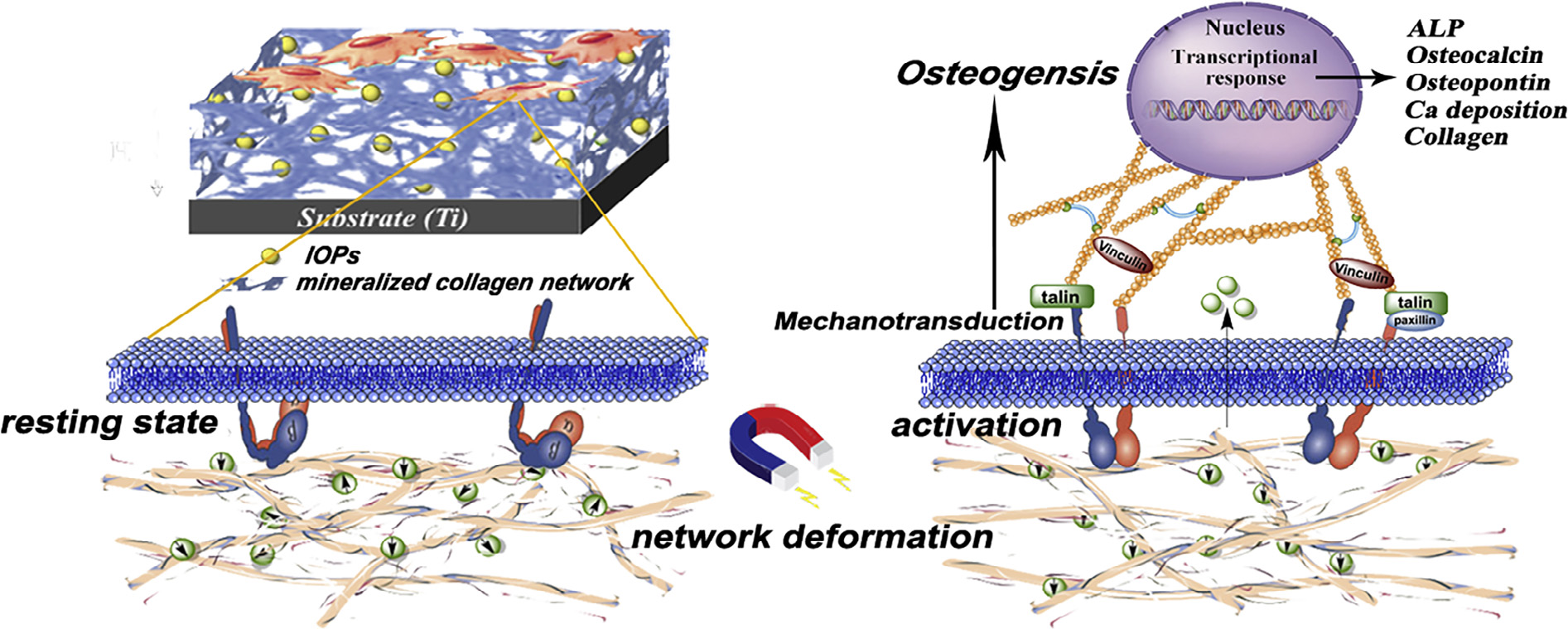
(Acta Biomaterialia xxx (2018) xxx–xxx ) 人们普遍认为通过在骨植入界面施加机械刺激可以激活细胞的力传导通道,从而提高初始的骨整合建立并保证植入物的临床成功。然而,控制骨植入界面处的机械刺激仍然存在一个挑战。 在这项研究中,浙江大学的翁文剑教授团队设计了一种磁响应涂层的策略,这是因为在静磁场(SMF)作用下涂层会变形,从而使磁响应涂层的机械刺激受到控制。通过电化学共沉积法在钛板上合成四氧化三铁纳米粒子/矿化胶原蛋白(IOP-MC)涂层,且不同涂层的IOP含量和分布有所不同;所得到的涂层被验证其具有与水凝胶相同柔韧性的溶胀性能。实验证明涂层中IOP的相对含量与分布在调解细胞行为中起着重要的作用。质量比为0.67且IOPs分布在IOP-MC涂层外层时(O-IOP-MC),细胞显示出最明显的促成骨分化活性,这可能归因于O-IOP-MC涂层暴露于SMF下使机械刺激最大化。此外, MC3T3-E1细胞受刺激后细胞成骨分化增强,其来源于磁驱动的机械转导信号通路,体现在成骨相关和力传导相关基因的表达均上调。因此这项工作提供了一个有希望的策略,对植入骨施加机械刺激以激活骨植入界面的力传导,从而促进骨整合。 Mechanical stimuli at the bone-implant interface are considered to activate the mechanotransduction pathway of the cell to improve the initial osseointegration establishment and to guarantee clinical success of the implant. However, control of the mechanical stimuli at the bone–implant interface still remains a challenge. In this study, Wenjian Weng working in the School of zhejiang University have designed a strategy of a magnetically responsive coating on which the mechanical stimuli is controlled because of coating deformation under static magnetic field (SMF). The iron oxide nanoparticle/mineralized collagen (IOP-MC) coatings were electrochemically codeposited on titanium substrates in different quantities of IOPs and distributions; the resulting coatings were verified to possess swelling behavior with flexibility same as that of hydrogel. The relative quantity of IOP to collagen and the IOP distribution in the coatings were demonstrated to play a critical role in mediating cell behavior. The cells present on the outer layer of the distributed IOP-MC (O-IOP-MC) coating with a mass ratio of 0.67 revealed the most distinct osteogenic differentiation activity being promoted, which could be attributed to the maximized mechanical stimuli with exposure to SMF. Furthermore, the enhanced osteogenic differentiation of the stimulated MC3T3-E1 cells originated from magnetically actuated mechanotransduction signaling pathway, embodying the upregulated expression of osteogenic-related and mechanotransduction-related genes. This work therefore provides a promising strategy for implementing mechanical stimuli to activate mechanotransduction on the bone–implant interface and thus to promote osseointegration. (郝莉莉) |