|
基质成分、微观结构及持续锶释放在诱导间充质干细胞的成骨分化中发挥拮抗作用
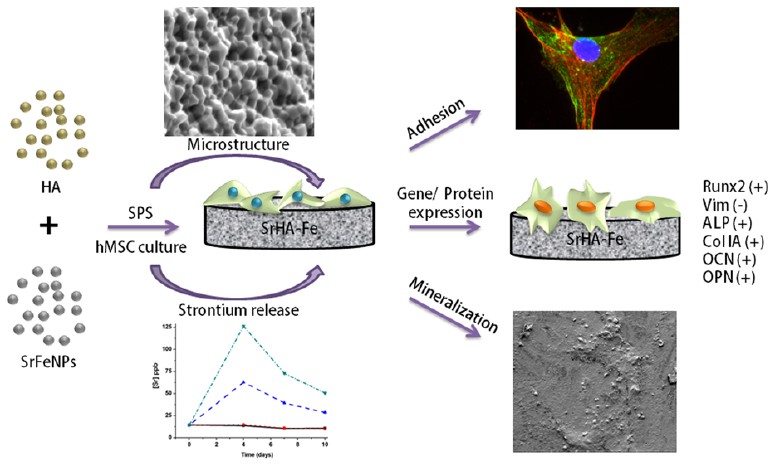
(ACS Appl. Mater. Interfaces 2017, 9, 19389−19408) 锶释放生物活性陶瓷是一类重要的治疗骨质疏松症的生物材料。Sr2 +与Ca2 +离子大小和极性相似性促进了两者在骨组织中离子交换.锶离子的生物学意义源于其在骨稳态中的作用,其可抑制破骨细胞活性。但第一原理计算结合同步加速数据的Rietveld精修示,与Ca(1)位点相比,当Ca(2)位点发生锶取代钙时,HA(P63 / m)晶格产生较大变形。 针对这个问题,印度材料研究中心生物材料实验室的Bikramjit Basu研究员团队设计了一种新的策略。在这项研究中,他们评估SrFe12O19纳米粒子的合成,相组装和磁性。在生物相容性方面, 研究了SrFe对hMSCs的细胞毒性与SrFe尺寸和剂量依赖性的关系。验证SrFe无毒本质后,他们以不同量(x=0,10和20wt%)的锶六角铁氧体纳米颗粒(SrFeNPs)与生物活性复合材料羟基磷灰石(HA)通过多级放电等离子烧结(SPS)技术进行固结。 Rietveld精修显示,放电等离子体烧结复合材料后SrFe12O19接近完全分解成磁铁矿(Fe3O4),同时HA的单胞体积增加,与锶掺杂的HA相对应。通过对干细胞分化的定性和定量形态学分析和表型与基因型表达,评估SrHA-Fe复合物与hMSC的细胞相容性。波形蛋白表达和成骨表型的获得物的减少,和骨诱导培养液中SrHA-Fe复合物上观察到磷酸酶(ALP)和胶原沉积,表明hMSCs的多能性显著降低。在SrHA-Fe复合材料上检测到成骨标记基因(Runx2,ALP和OPN)显著上调,但OCN和Col IA表达与HA同样高。然而,SrHA-Fe复合材料上基质矿化的增加与Sr2 +和Fe2 +的释放相对应。总而言之,目前的工作是对锶六铁氧体作为无毒纳米生物材料的第一次报导。此外,与纯HA相比,以SrHA为基的铁氧复合材料可以更好的促进骨形成。 Strontium releasing bioactive ceramics constitute an important class of biomaterials for osteoporosis treatment. The chemical similarity of Sr2+ to Ca2+ in terms of ionic size and polarity facilitates easy ion exchange of Sr2+ with Ca2+ in the bone tissue. The biological significance of strontium ion (Sr2+) stems from its role in bone homeostasis, wherein it inhibits osteoclast activity and encourages osteoblast growth and differentiation.First-principle calculations combined with Rietveld refinement of synbstitution revealed a greater distortion of the HA (P63/m) lattice, when calcium substitution by strontium occurs at the Ca(2) site as compared to Ca(1) site. In term of this issue, Bikramjit Basu working in Laboratory for Biomaterials, Materials Research Centre, India have designed a new strategy. In this study,They evaluated the synthesis, phase assemblage, and magnetic properties of strontium hexaferrite, SrFe12O19, (SrFe) nanoparticles. On the biocompatibility front, the size- and dosedependent cytotoxicity of SrFe against human mesenchymal stem cells (hMSCs) were investigated. After establishing their non-toxic nature, they used the strontium hexaferrite nanoparticles (SrFeNPs) in varying amount (x = 0, 10, and 20 wt %) to consolidate bioactive composites with hydroxyapatite (HA) by multi-stage spark plasma sintering (SPS). Rietveld refinement of these spark plasma sintered composites revealed a near complete decomposition of SrFe12O19 to magnetite (Fe3O4) along with a marked increase in the unit cell volume of HA, commensurate with strontium-doped HA. The cytocompatibility of SrHA-Fe composites with hMSCs was assessed using qualitative and quantitative morphological analysis along with phenotypic and genotypic expression for stem cell differentiation. A marked decrease in the stemness of hMSCs, indicated by reduced vimentin expression and acquisition of osteogenic phenotype, evinced by alkaline phosphatase (ALP) and collagen deposition was recorded on SrHA-Fe composites in osteoinductive culture. A significant upregulation of osteogenic marker genes (Runx2, ALP and OPN) was detected in case of the SrHA-Fe composites, whereas OCN and Col IA expression were similarly high for baseline HA. However, matrix mineralization was elevated on SrHA-Fe composites in commensurate with the release of Sr2+ and Fe2+. Summarizing, the current work is the first report of strontium hexaferrite as a non-toxic nanobiomaterial. Also, SrHA-based iron oxide composites can potentially better facilitate bon formation, when compared to pristine HA. (郝莉莉)
|