|
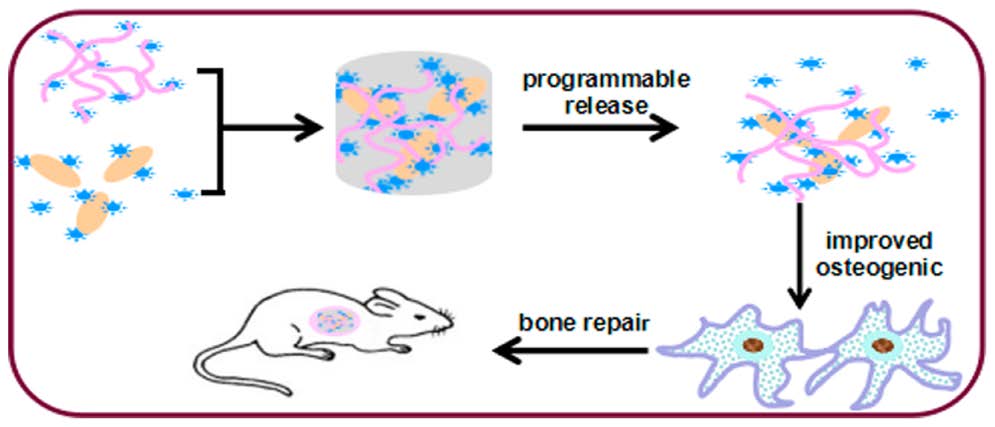
用于骨修复的可调控的生长因子释放SF/HA纳米级支架
ACS Appl. Mater. Interfaces 2016, 8, 24463−24470 对于修复大量骨缺损,骨诱导生物材料很具有吸引力,并且具有骨诱导能力骨支架的仿生是起很大作用的。来自苏州大学的Ding等研究人员改进了仿生设计优化了蚕丝蛋白(SF)和羟基磷灰石(HA)组成的复合支架的成骨能力。SF纤维和水分散的HA纳米粒子混合均匀后制备成纳米级的复合支架,其中含有40%的高含量的HA来模拟骨基质。骨形态发生蛋白-2(BMP2)被粘附到SF-HA复合支架上来调节其释放。在体外的研究表明复合支架上的BMP2保留了生物活性,并且能够通过调节BMP2粘附在SF-HA上的比例来调节其持续释放。体外和体内的成骨实验都表明复合支架在适当的BMP2释放条件下,显示出了优越的成骨能力,与之前报道过的粘附BMP2的SF-HA复合支架相比有显著提高。因此,这种粘附有可调控BMP2释放能力的仿生SF-HA纳米级支架为骨再生提供了更好的微环境。 Osteoinductive biomaterials are attractive for repairing a variety of bone defects, and biomimetic strategies are useful toward developing bone scaffolds with such capacity. Here, Ding et al. from Soochow University developed a multiple biomimetic design to improve the osteogenesis capacity of composite scaffolds consisting of hydroxyapatite nanoparticles (HA) and silk fibroin (SF). SF nanofibers and water-dispersible HA nanoparticles were blended to prepare the nanoscaled composite scaffolds with a uniform distribution of HA with a high HA content (40%), imitating the extracellular matrix (ECM) of bone. Bone morphogenetic protein-2 (BMP-2) was loaded in the SF scaffolds and HA to tune BMP-2 release. In vitro studies showed the preservation of BMP-2 bioactivity in the composite scaffolds, and programmable sustained release was achieved through adjusting the ratio of BMP-2 loaded on SF and HA. In vitro and in vivo osteogenesis studies demonstrated that the composite scaffolds showed improved osteogenesis capacity under suitable BMP-2 release conditions, significantly better than that of BMP-2 loaded SF−HA composite scaffolds reported previously. Therefore, these biomimetic SF−HA nanoscaled scaffolds with tunable BMP-2 delivery provide preferable microenvironments for bone regeneration. (莫莉)
|