Biomaterials 287 (2022) 12166
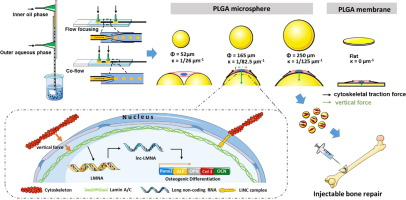
微球载体作为细胞治疗所需的用于培养/扩增组织细胞的三维基质引起了极大的兴趣。然而,微球曲率如何影响细胞增殖/分化以及干细胞的潜在信号通路,以及如何通过操纵微载体表面曲率来调节这些细胞功能,仍有待探索。因此,目前的研究旨在开发一种微流体操纵技术,以精确控制聚乳酸-乙醇酸 (PLGA) 微球表面曲率,并随后研究大鼠骨间充质干细胞 (BMSCs) 的细胞反应和反应途径。在这些具有预定曲率的微球上培养。-1至 1/125 µm -1。对BMSCs的附着和增殖进行了评估,发现κ=1/82.5 µm -1之一为细胞生长和成骨分化提供了最合适的微环境。甚至发现κ=1/82.5 µm -1的微载体上的细胞显着增强了F-肌动蛋白的细胞骨架组织、核变形和Lamin A的表达。 此外,本研究发现一种名为 lnc-LMNA 的长链非编码 RNA 是与 Lamin A 相关的关键因素,可调节 BMSCs 在球形基质上的成骨分化。因此,目前的研究通过微流控制造微载体提供了一种智能操作技术来调节细胞功能,从而增强基于细胞的再生或修复的预期治疗效果。
Microsphere-carriers have gained great interests as three-dimensional substrates for cultivation/expansion of tissue cells, required by cell-based therapy. However, how the microsphere curvature affects the cell proliferation/differentiation as well as the underlying signaling pathway of stem cells, and how to consequently regulate those cellular functionalities via manipulating microcarrier surface curvature, still remain tobe explored. The current study was thus designed to develop a microfluidic manipulation technology to precisely control poly (lactic-co-glycolic) acid (PLGA) microsphere surface curvature, and subsequently to investigate the cellular responses and responding pathways of rat bone mesenchymal stem cells (BMSCs) cultured on these microspheres of predetermined curvature. A microfluidic device was developed to produce mono-distributed PLGA microspheres of diameters ranging from 52 µm to 250 µm, corresponding to curvatures (κ) from 1/26 µm−1 to 1/125 µm−1. BMSCs attachment and proliferation was evaluated on them and the one of κ = 1/82.5 µm−1 was shown to provide the most suitable microenvironment for cells to grow and undergo osteogenic differentiation. It was even found that F-actin cytoskeletal organization, nuclear distortion and expression of Lamin A were significantly enhanced by cells on the microcarriers of κ = 1/82.5 µm−1. Furthermore, a long non-coding RNA named lnc-LMNA, was found in this study to be the key factor associated with Lamin A to regulate osteogenic differentiation of BMSCs on spherical substrates. The current study thus provides a smart manipulation technology via microfluidic-manufacturing microcarriers to regulate cell functionalities, thereby enhancing desired therapeutic outcomes of cell-based regeneration or repair.
周小松