 Biomaterials, 2020, 120357
Biomaterials, 2020, 120357
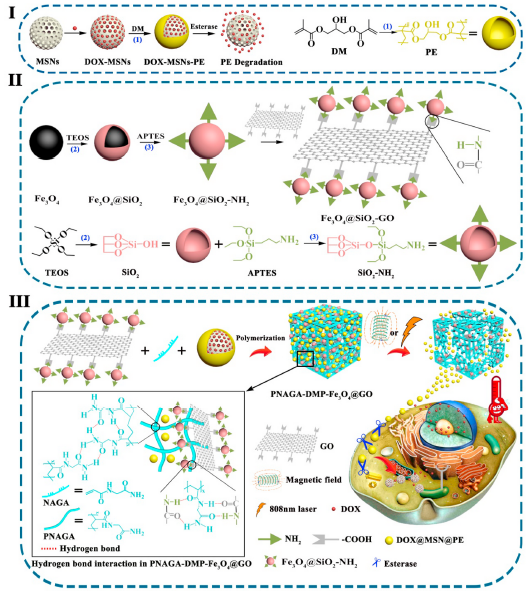
化学疗法是治疗恶性肿瘤最常用的方法之一。然而,能够根据肿瘤治疗需求精确地操纵局部药物释放的可控化学疗法仍然是一个挑战。近日,西安交通大学陈新教授报道了一种磁光双响应的水凝胶,其结合了热敏的聚丙烯酰甘氨酰胺(PNAGA),担载阿霉素(DOX)和聚酯(PE)包裹的介孔二氧化硅纳米载体(MSN)以及Fe3O4纳米颗粒(Fe3O4 NPs)接枝修饰的氧化石墨烯(GO)来解决上述问题。 Fe3O4 NPs和GO分别作为磁热剂和光热剂进行热疗,同时在不同的磁场和/或近红外辐照条件下可产生不同程度的PNAGA链运动。该策略不仅使水凝胶通过预先加热实现了凝胶-溶胶的转变从而进行肿瘤注射,而且还实现了DOX-MSNs-PE(简称DMP)纳米载体的可控释放途径,以满足不同患者和不同肿瘤状态的不同需求。此外,这些释放的DMP纳米载体可被周围的肿瘤细胞胞吞,然后在酯酶触发PE快速水解后将药物传递至这些细胞,从而实现精准化疗。体外和体内实验结果均表明PNAGA-DMP-Fe3O4@GO水凝胶结合了可控的化疗和热疗,在小鼠模型中可清除90%以上的肿瘤细胞并有效抑制肿瘤生长,表明本研究制备的水凝胶是实现精准肿瘤治疗的绝佳候选者。
Chemotherapy is one of the most commonly utilized approaches to treat malignant tumor. However, the well-controlled chemotherapy able to accurately manipulate local drugs for on-demand tumor treatment is still a challenge. Recently, Professor Chen Xin from Xi’an Jiao Tong University reported a magnet and light dual-responsive hydrogel combining thermosensitive poly(N-acryloyl glycinamide) (PNAGA), doxorubicin (DOX) loaded and polyester (PE) capped mesoporous silica nanocarriers (MSNs) as well as Fe3O4 nanoparticles (Fe3O4 NPs) grafted graphene oxide (GO) was fabricated to address above issue. The Fe3O4 NPs and GO respectively serve as magnetothermal agent and photothermal agent to perform hyperthermia, meanwhile to generate chain motion of PNAGA with varying degrees under different conditions of magnetic field and/or NIR irradiation. This strategy not only allowed the gel-sol transition of the hydrogel by prior heating for tumor injection, but performed controllable release routes of DOX-MSNs-PE (DMP for short) nanocarriers to meet various requirements from different patients and the changing states of tumor. Furthermore, these escaped DMP nanocarriers could be taken by surrounding tumor cells, and then deliver their drug to these cells after rapid hydrolysis of the PE cap triggered by esterase, resulting in accurate chemotherapy. Both in vitro and in vivo results indicated that the PNAGA-DMP-Fe3O4@GO hydrogel combining well-controllable chemotherapy and hyperthermia can eliminate more than 90% tumor cells and effectively inhibit the tumor growth in mice model, demonstrating the great candidate of our hydrogel for accurate tumor therapy.
DOI:10.1016/j.biomaterials.2020.120357
(郝莉莉)