ACS Appl. Mater. Interfaces 2020, 12, 14, 16097–16103
仿生微载体作为细胞培养模型已经被广泛应用,其优点就是较大的比表面结和良好的生物相容性。通常的制备方法有电纺丝、激光蚀刻、微流控技术等。但要模仿天然组织,操控需要非常的精细、可控与准确,所以很难用简单的方法实现。简单的球形微载体形状限制了其在特殊组织结构(例如血管组织等)中的进一步应用。通过简易、方便的办法制备具有特殊结构和功能的新型微载体是非常必要的。利用微流控纺丝仿生制备的新型螺旋微丝可称之为螺旋微马达。制备微马达的方法通常有,蚀刻技术、电沉积技术、倾斜沉积技术、激光扫描和模板辅助等方法,但通常需要尖端的设备工艺和相对较高的成本,而且局限于固定的材料,制备的尺寸比较小,很难进行细胞培养。方法简单,价格低廉的微马达制备方法仍然很欠缺。
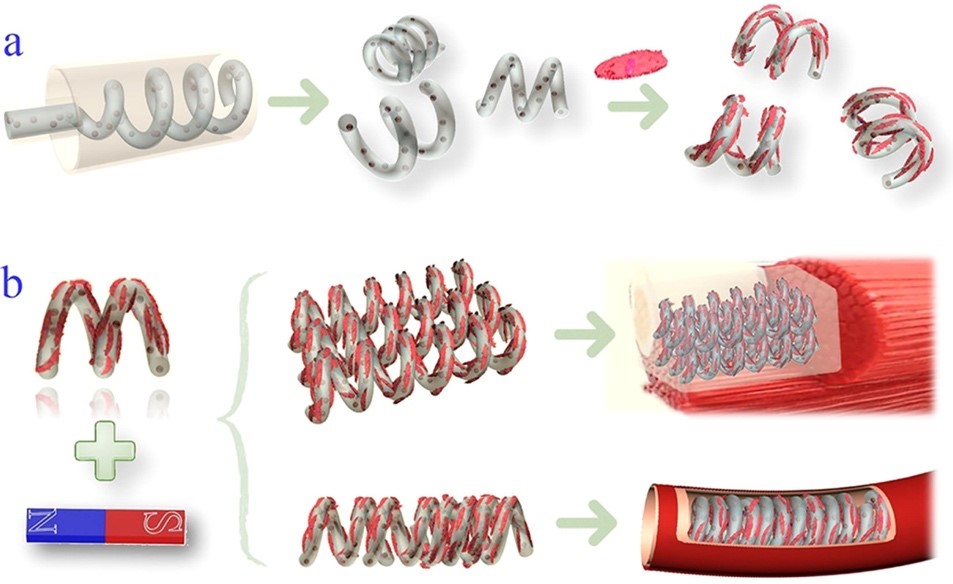
近日,东南大学赵远锦教授受到细菌鞭毛的螺旋旋转和平移运动的启发,通过微流控纺丝和缠绕技术制备了具有磁性的螺旋微马达作为动态细胞微载体。该方法简便、易控,可以通过流速和切割距离对微马达进行调整。与现有的制备方法相比,利用微流控技术可以相对简单和低廉地,在微米级连续地制备具有预期的3D形状和材料组成的微载体。仅需要选择一种生物相容性材料同时能够满足螺旋微丝的产生与细胞培养即可。由于具有特殊的结构同时含有磁性纳米粒子,螺旋微马达可以在低磁场强度下进行评平移和旋转。担载细胞的螺旋微马达可以进行迁移、聚集并组装成具有不同几何形貌的结构。同时可以进一步包埋于具有生物相容性的水凝胶中用于组织修复,还可以用作血管支架和血管模型进行器官芯片的研究。动态细胞微载体扩展了其在生物医学领域的应用。
Microcarriers have emerged in a vast number of biomimetic building blocks that were created as cell culture models. Benefiting from their large surface-to-volume ratio and good matrix biocompatibility, microcarriers could not only offer a high surface area for cell attachment and cultivation but also provide complex microenvironment conditions that mimic natural in vivo three-dimensional (3D) structures for the transportation of nutrients and metabolites and for cellular interactions as well. Owing to these features, numerous cell microcarriers have been created using electrospray, laser lithography, microfluidics, etc. However, to construct complex constructions resembling natural tissues, these microcarriers were usually needed to be manipulated elaborately, controllably, and precisely, which is difficult to realize through simple approaches. Moreover, most of the microcarriers were of simple spherical shapes that impeded their further assembly into specific tissue structures such as vascular structures. Thus, novel microcarriers with unique structures and functions that can be assembled in a simple and convenient way are still highly expected. However, most of the recently developed micromotors are fabricated from lithography, electrodeposition, glancing angle deposition, laser scanning, template-assisting methods, etc. These methods include steep instrumental demands and relatively high expenses during the fabrication process. Moreover, the resultant micromotors are restricted to certain materials that should be suitable for the fabrication method and are often with small sizes, which makes their use during cell cultivation difficult. These indicate that a relatively simple and low-cost fabrication method for micromotors that could be applied as versatile cell culture blocks and tissue regeneration modules is still deficient.
Recently, Professor Zhao Yuanjin from Southeast University of Biological and Science and Medical Engineering was inspired by the helical rotational and translational motion of bacterial flagella, they presented novel helical micromotors as dynamic cell microcarriers using a microfluidic-spin helical microfiber template. Benefiting from the precise control of fluids in microfluidic channels and the dicing process, the morphologies of the magnetically helical microfiber templates as well as helical micromotors could be easily tailored by tuning flow rates and cutting distances. Compared to the existing approaches, this fabrication method could consecutively produce micromotors with desired 3D shapes and material compositions at the microscale in a relatively simple and low-cost manner, which does not need demanding instrumental manipulations or strict experimental environments. Thus, by choosing a typical biocompatible material that is suitable for both helical microfiber generation and cell cultivation, the resultant helical micromotors are designed as ideal cell microcarriers. Due to the specific structure and magnetic nanoparticle encapsulation, the helical micromotors could perform paralleling movement and rotated locomotion in response to a corresponding low-strength magnetic field. These enable the cell-enriched helical micromotors to migrate, aggregate, and assemble into different geometrical constructions including planar arrangements and tubes. The cell-seeding microcarrier stack could be further embedded into a biocompatible hydrogel to form a cell block that is useful for damaged tissue repair, while the tube-structured cell-seeding microcarrier assembly could be used as blood vessel scaffolds or vascular models for organ-on-chip study fields. All of these features prove the developed micromotors to be used as dynamic cell microcarriers, which would broaden their biomedical application scope.
https://doi.org/10.1021/acsami.0c01264
孙烁