ACS Appl. Mater. Interfaces 2020, 12, 45891−45903
骨肉瘤是最常见的间叶组织来源的原发性恶性骨肿瘤,多见于儿童和青少年。目前临床治疗手段主要依靠术前辅助化疗、手术切除并植入人工假体来填补骨缺损和术后化疗。遗憾的是,人工假体的成骨能力不足、化学疗法产生患者难以忍受的副作用及康复期间可能发生的感染事件,都会不同程度上影响患者术后效果并加重患者经济和心理负担。聚醚醚酮(PEEK)因其力学性能与人皮质骨相似的优势逐渐成为骨植入物的热门候选材料。然而,PEEK固有的生物惰性也严重限制了其在临床上的广泛应用。以往对PEEK材料的研究主要集中在如何提高其骨整合和抗菌性能,而对PEEK材料表面抗肿瘤方面的关注较少。因此,赋予PEEK植入物抗肿瘤性能、增强的骨整合和抵抗细菌感染性能在骨肿瘤外科领域具有较大潜力。
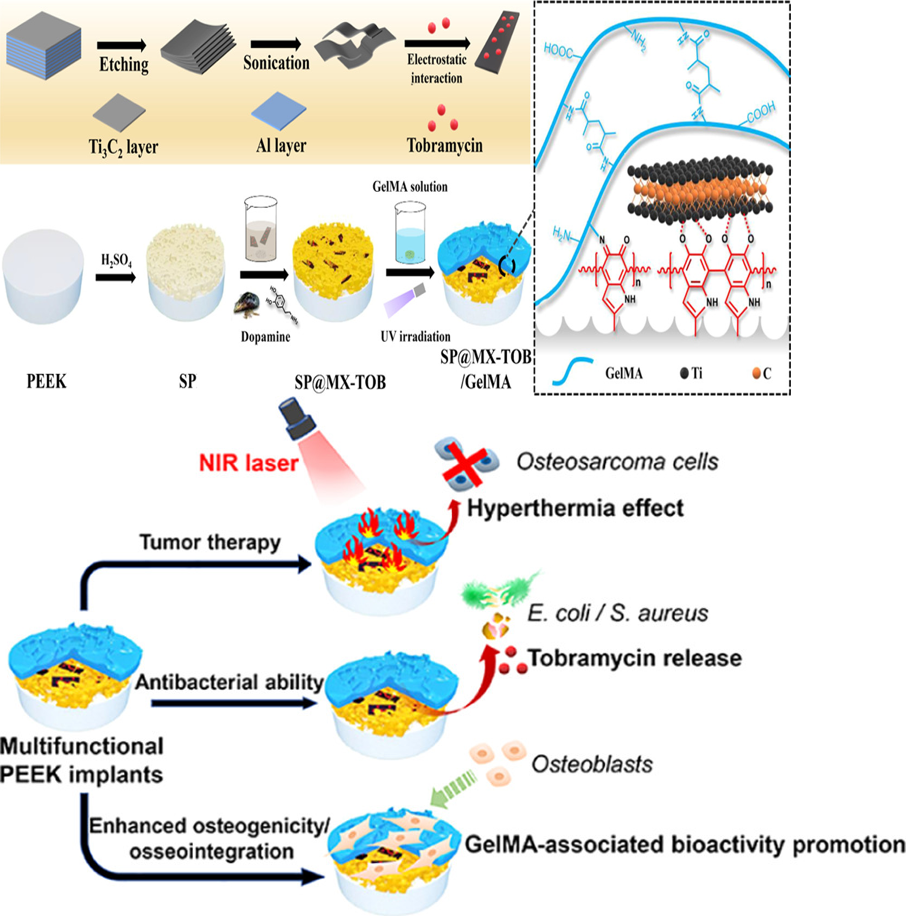
近日,四川大学化学工程学院邓怡团队报道了一种具有抗肿瘤、抑菌和成骨三功能的聚醚醚酮植入物表面,即通过聚多巴胺—钛金属元素的金属配位络合作用将搭载有妥布霉素的新型二维材料MXene修饰在多孔磺化聚醚醚酮表面,随后借助迈克尔加成或席夫碱反应将明胶甲基丙烯酸酯水凝胶固定到材料表面。一方面,MXene和聚多巴胺产生的协同光热效应,能够在808 nm近红外辐射下通过热消融有效杀死多功能涂层上的骨肉瘤细胞;另一方面,装载的妥布霉素对革兰氏阴性/革兰氏阳性细菌均显示出强大的抗菌性能。更重要的是,多功能植入物还具有优异的细胞相容性和促成骨能力。因此,这种光热控制的多功能植入物不仅可以杀死骨肉瘤细胞和细菌,还能够增强骨整合,这对于治疗骨肉瘤切除术后的骨缺损具有关键作用。
After an osteosarcoma resection, the risks of cancer recurrence, postoperative infection, and large bone loss still threaten patients’ health. Conventional treatment relies on implanting orthopedic materials to fill bone defects after surgery,but it has no ability of destroying residual tumor cells and preventing bacterial invasion. Recently, to tackle this challenge, an interesting report by Deng Yi and team from Sichuan University of School of Chemical Engineering developed a novel multifunctional implant (SP@MX/GelMA) that mainly consists of MXene nanosheets, gelatin methacrylate (GelMA) hydrogels, and bioinert sulfonated polyetheretherketone (SP) with the purpose of facilitating tumor cell death, combating pathogenic bacteria, and promoting osteogenicity. Because of the synergistic photothermal effects of MXene and polydopamine(pDA), osteosarcoma cells are effectively killed on the multifunctional coatings under 808 nm near-infrared (NIR) irradiation through thermal ablation. After loading tobramycin (TOB), the SP@MX-TOB/GelMA implants display robust antibacterial properties against Gram-negative/Gram-positive bacteria. More importantly, the multifunctional implants are demonstrated to have superior cytocompatibility and osteogenesis-promoting capability in terms of cell replication, spreading, alkaline phosphatase activity, calcium matrix mineralization, and in vivo osseointegration. Accordingly, such photothermally controlled multifunctional implants not only defeat osteosarcoma cells and bacteria but also intensify osteogenicity, which hold a greatly promising countermeasure for curing postoperative tissue lesion from an osteosarcoma excision.
DOI: doi.org/10.1021/acsami.0c14752
高承哲