Chen AZ.Small. 2019 Jun;15(25):e1901397.
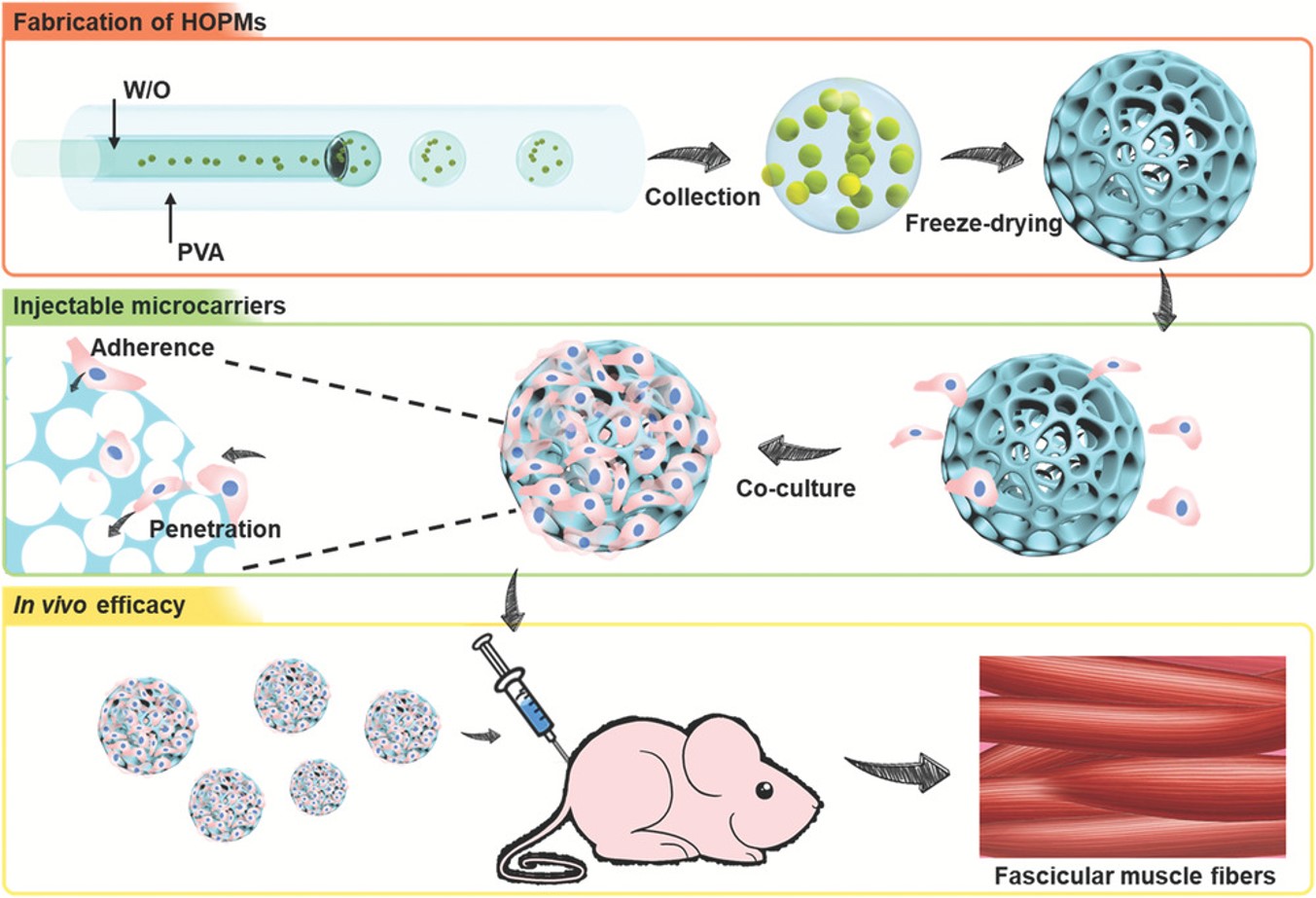
微尺度细胞载体最近在修复组织缺损方面获得了极大的兴趣,因为它避免了使用植入物进行组织再生的大规模开放手术。华侨大学陈爱政教授课题组利用微流体技术制备了高开放的多孔微球,用于包藏增殖的骨成肌细胞,并评估了其在原位传递细胞的可行性。这些具有生物相容性的HOPMs颗粒大小为280370 m,具有1080 m的开放孔和相互连通的路径。HOPMs的这种结构方便地提供了良好的微环境,细胞与细胞外基质紧密排列成细长的形状,促进细胞粘附和增殖,增强肌原性分化。此外,小鼠体内实验结果证实,细胞保留和血管化以及部分成肌细胞分化得到改善。这些模块化细胞负载微载体可能允许在微创输送后进行原位组织构建,为再生医学提供了一种方便的手段
Microscale cell carriers have recently garnered enormous interest in repairing tissue defects by avoiding substantial open surgeries using implants for tissue regeneration. In this study, the highly open porous microspheres (HOPMs) are fabricated using a microfluidic technique for harboring proliferating skeletal myoblasts and evaluating their feasibility toward cell delivery application in situ. These biocompatible HOPMs with particle sizes of 280–370 µm possess open pores of 10–80 µm and interconnected paths. Such structure of the HOPMs conveniently provide a favorable microenvironment, where the cells are closely arranged in elongated shapes with the deposited extracellular matrix, facilitating cell adhesion and proliferation, as well as augmented myogenic differentiation. Furthermore, in vivo results in mice confirm improved cell retention and vascularization, as well as partial myoblast differentiation. These modular cell‐laden microcarriers potentially allow for in situ tissue construction after minimally invasive delivery providing a convenient means for regeneration medicine
DOI:10.1002/smll.201901397
周小松